|
|
Ultrasound Contrast Agents (Microbubbles) in the Microvasculature
Center for Industrial and Medical Ultrasound
|
What are we doing?
|
|
Why are we doing it?
|
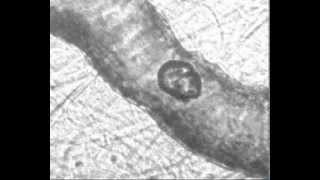
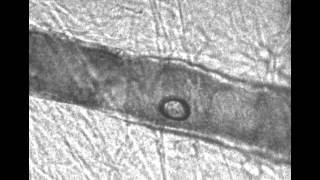
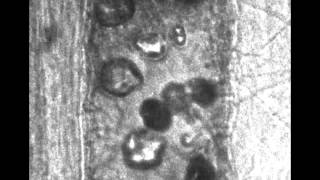
High-speed images of oscillating micro-bubbles in small blood vessels are imaged to observe how the bubble oscillations might help induce permeation in the endothelium, allowing drugs to be transported across that barrier.
|
|
Drugs and genes can be delivered locally using focused ultrasound. This can significantly improve uptake of molecules economically. Knowing just how these bubbles can open the endothelium barrier is thus important.
|
|
How are we doing it?
|
|
Specifics
|
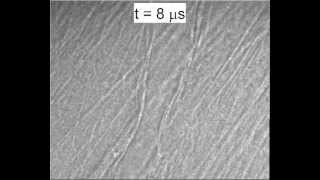
We excise the mesentery, immerse it in a Krebs solution and place it on a microscope. A flash lamp is used to deliver enough light to obtain good images with 50 nsec exposure times. Microbubbles are perfused along with a saline solution. When a vessel is found containing microbubbles, the experiment is triggered, sending a very short ultrasound pulse (1 MHz) towards the tissue sample. 14 images are collected at pre-determined times (usually every 150 or 300 nsec). Quantification of the images gives us information about vessel deformations, bubble oscillations, and registration of the specific locations that are later used to correlate vessel motion with histological observations of vessel damage.
|
|
The experimental setup and timing diagram are illustrated in the following figure:
|
|
|
|
Two important notes
|

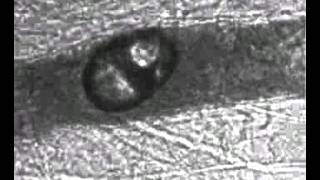
Vessel size: 22 µm
Pressure amplitude: 1.8 MPa
|
|
- Acoustic pulses come from out of the page. The observed microbubble and microvessel motions are orthogonal to the acoustic directions. Thus, the effects are NOT due to acoustic radiation force.
- Image sequences are taken on sub-microsecond timescales. Vessel response is forced, it is not an evoked response.
|
|
|
© 2011–2013 University of Washington
|
|
|
|
Conclusion
|
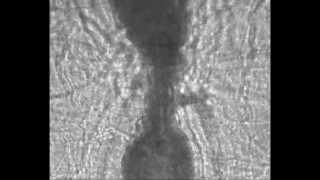
In compliant microvessels, like venules, arterioles and capillaries, a major effect is that the microvessel wall gets pulled into the lumen, which we call invagination.

Invagination in a 50-µm vessel under 6 MPa peak negative pressure.
|
|
For microvessels that aren’t too small, invagination exceeds distention, suggesting that invagination may be a dominant mechanism for vascular bioeffects, especially in microvessels larger than about 20 µm.
|
|
|
|
|
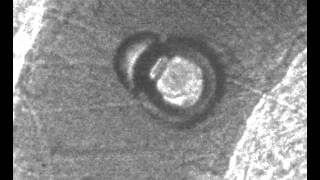
Microbubbles growing and collapsing. When they collapse, the vessel wall gets pulled into the lumen. Pressure amplitude = 7 MPa, vessel diameter = 46 µm.
Invagination in a 50-µm vessel under 6 MPa peak negative pressure.
Microbubble collapse leading to invagination in a 53-µm vessel
insonified at 7 MPa.
|
|
|
|
|
© 2011–2013 University of Washington
|
|
|
Distention is important in relatively small microvessels.
|
|
Microbubble/vessel interactions for relatively large and small microvessels
|
|
|
|
|
|
|
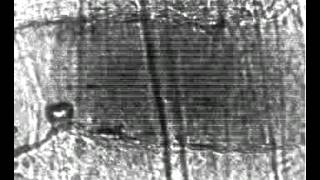
Large vessel, high pressure (7.2 MPa):

Image sequence and selected images of microbubbles insonified at 7.2 MPa peak negative pressure in a 100 µm diameter microvessel.
A relatively large vessel (100 µm diameter) responds to a forced oscillation of microbubbles. The vessel’s major response is invagination.
|
|
Small vessel, high pressure (7.2 MPa):

Image sequence and selected images of microbubbles insonified at 7.2 MPa peak negative pressure. Vessel diameter is 17 µm.
A relatively small vessel (17 µm diameter) can be ruptured by the forced oscillation of microbubbles under relatively high pressure (7.2 MPa). The vessel responds with both distention (max at 0.6 µs) and invagination (see image at 1.05 µs). Damage is obvious, as re-growth of microbubbles is mostly outside original vessel (see image at 1.2 µs).
|
|
Conclusion
|
Vascular effects depend on relative pressure AND relative vessel size.
|
|
|
© 2011–2013 University of Washington
|
|
Effect of proximity between microbubble and vessel wall
|
|
|
|
|
|
Conclusion
|
Microbubble/vessel interactions in a 71-µm diameter vessel and 4.3-MPa peak negative pressure.
|
|
The microbubble on the left is close to the lower vessel wall, and the interaction is dramatic. The microbubble is probably jetting away from the vessel wall, and “pulling” the wall with it. The degree and curvature of invagination may be sufficient to induce vascular bioeffects. The microbubble to the right is not close, and the interaction is minimal. This suggests why vascular damage is not prominent with microbubbles: They are mostly constrained to the center of vessels (due to parabolic flow conditions).
|
|
|
© 2011–2013 University of Washington
|
|
|
|
Conclusions
|

Microbubble subjected to a 4-MPa amplitude pulse in a 15-µm vessel. Jet observed in frame 4. Jet propagates from top to bottom, poking a hole through the vessel wall, ending in the tissue.
Microbubble response subject to a 4.3-MPa peak negative pressure amplitude in a 77-µm vessel. Max distention = 1 µm, max invagination = 7 µm.
|
|
- Many researchers presume that vascular damage occurs from a liquid jet impinging on the vessel wall. In our experiments, the jetting direction was ALWAYS away from the vessel wall, not towards it. This is probably due to the compliance of these particular vessels.
- Although the jetting direction was ALWAYS away from the vessel wall, if the vessel is small enough, then the jet can impact the opposite wall and cause bioeffects.
|
|
|
© 2011–2013 University of Washington
|
|
|
|
Conclusion
|
Microbubble oscillation causes deformation of the vessel wall, and in turn is affected by that deformation.These coupled interactions can generate translational motion of a microbubble. Below are additional image sequences showing microbubble translation caused by the elastic response from the deformed vessel wall.
|
|
In these compliant microvessels, microbubble translation is AWAY from the vessel wall. This suggests that (1) bioeffects associated with microbubbles will be reduced because they quickly (in 1 cycle) translate away from the vessel wall, and (2) targeted microbubbles will feel a force pulling them away from the vessel wall. This force should be accounted for when considering the strength of adhesion studies.
|
|
|
|
|
Microbubble translation in a 50-µm vessel being insonified by 4.3 MPa.
This image sequence shows translation of many microbubbles. Microbubble–microbubble interactions may be the dominant mechanism for “pulling” the bubbles away from the vessel wall and towards the centerline of the vessel.
|
|
|
|
|
© 2011–2013 University of Washington
|
|
|
|
Conclusion
|
Given the exact microbubble position, we can determine if there have been any bioeffects by performing histology or TEM on the tissue samples.
|
|
We have been able to correlate microbubble dynamics with vessel deformation and vascular bioeffects from the exact same location. Interestingly, very little “damage” is observed. Many tissue samples didn’t show any damage, and no damage has been observed (for a single cycle pulse) that extends past the basement membrane. We conclude that damage probably occurs over repeated insonations.
|
|
|
|
|

(a) Microbubble dynamics in a 33-µm venule insonified at 1.5 MPa. A time stamp for each frame is located at the bottom and the scale bar on the bottom right of the last frame. Dashed lines highlight the locations of the vessel wall in the first and last frames. (b) Measurement of vessel wall displacements. The displacements were measured at the same point (indicated by the arrow in each frame) on the vessel wall that had the largest extent of deformation. This example shows that MB oscillation induced the distention and invagination of both sides of the vessel wall with invagination significantly larger than distention.
|
|

Corresponding histology. (a) Control section obtained from the same venule, several mm away. It shows the vessel section was intact. (b) A representative section within the targeted region. The dark material (arrow) inside the vessel lumen appears to form a tag of tissue that is continuous with the vessel wall, suggestive as being part of an endothelial cell (nucleus and some cytoplasm) that was torn free from the vessel wall.
|
|
|
© 2011–2013 University of Washington
|
|
Relaxation of vessel wall
|
|
Conclusion
|
After the microbubble stops oscillating (a couple of µs), the invaginated tissue relaxes back to its equilibrium position. Knowledge of the relaxation rate gives some information about the viscoelastic properties of the tissue. We measure the relaxation time, and use a standard Voigt model to fit the relaxation data.
|
|
Microbubble dynamics generate strain rates that have, until now, not been studied. At these higher pressures, the initial strains are also quite high.
|
|
|
|
|
|
|
|

Data captured from the image sequence, fitted with the Voigt viscoelastic tissue model. The relaxation time constant τ = the ratio of viscosity to elasticity of the tissue (= η/E).
|
|
|
© 2011–2013 University of Washington
|
|
|
 |
|
Preliminary observations on the spatial correlation between short-burst microbubble oscillations and vascular bioeffects Chen, H., A.A. Brayman, A.P. Evan, and T.J. Matula, "Preliminary observations on the spatial correlation between short-burst microbubble oscillations and vascular bioeffects," Ultrasound Med. Biol., 12, 2151-2162, doi:10.1016/j.ultrasmedbio.2012.08.014, 2012. |
|
More Info
| |
|
1 Dec 2012 
|
|
 |
|
|
The objective of this preliminary study was to examine the spatial correlation between microbubble (MB)-induced vessel wall displacements and resultant microvascular bioeffects. MBs were injected into venules in ex vivo rat mesenteries and insonated by a single short ultrasound pulse with a center frequency of 1 MHz and peak negative pressures spanning the range of 1.5%u20135.6 MPa. MB and vessel dynamics were observed under ultra-high speed photomicrography. The tissue was examined by histology or transmission electron microscopy for vascular bioeffects. Image registration allowed for spatial correlation of MB-induced vessel wall motion to corresponding vascular bioeffects, if any. In cases in which damage was observed, the vessel wall had been pulled inward by more than 50% of the its initial radius. The observed damage was characterized by the separation of the endothelium from the vessel wall. Although the study is limited to a small number of observations, analytic statistical results suggest that vessel invagination comprises a principal mechanism for bioeffects in venules by microbubbles.
|
|
 |
|
Characteristic microvessel relaxation timescales associated with ultrasound-activated microbubbles Chen, H., A.A. Brayman, and T.J. Matula, "Characteristic microvessel relaxation timescales associated with ultrasound-activated microbubbles," Appl. Phys. Lett., 101, 163704, doi:10.1063/1.4761937, 2012. |
|
More Info
| |
|
15 Oct 2012 
|
|
 |
|
|
Ultrasound-activated microbubbles were used as actuators to deform microvessels for quantifying microvessel relaxation timescales at megahertz frequencies. Venules containing ultrasound contrast microbubbles were insonified by short 1 MHz ultrasound pulses. Vessel wall forced-deformations were on the same microsecond timescale as microbubble oscillations. The subsequent relaxation of the vessel was recorded by high-speed photomicrography. The tissue was modeled as a simple Voigt solid. Relaxation time constants were measured to be on the order of ~10 µs. The correlation coefficients between the model and 38 data sets were never lower than 0.85, suggesting this model is sufficient for modeling tissue relaxation at these frequencies. The results place a bound on potential numerical values for viscosity and elasticity of venules.
|
|
 |
|
Observations of translation and jetting of ultrasound-activated microbubbles in mesenteric microvessels Chen, H., A.A. Brayman, W. Kreider, M.R. Bailey, and T.J. Matula, "Observations of translation and jetting of ultrasound-activated microbubbles in mesenteric microvessels," Ultrasound Med. Biol., 37, 2139-2148, doi:10.1016/j.ultrasmedbio.2011.09.013, 2011. |
|
More Info
| |
|
1 Dec 2011 
|
|
 |
|
|
High-speed photomicrography was used to study the translational dynamics of single microbubbles in microvessels of ex vivo rat mesenteries. The microbubbles were insonated by a single 2 microsecond ultrasound pulse with a center frequency of 1 MHz and peak negative pressures spanning the range of 0.8-4 MPa. The microvessel diameters ranged from 10-80 micrometers. The high-speed image sequences show evidence of ultrasound-activated microbubble translation away from the nearest vessel wall; no microbubble showed a net translation toward the nearest vessel wall. Microbubble maximum translation displacements exceeded 20 micrometers. Microjets with the direction of the jets identifiable were also observed; all microjets appear to have been directed away from the nearest vessel wall. These observations appear to be characteristic of a strong coupling between ultrasound-driven microbubbles and compliant microvessels. Although limited to mesenteric tissues, these observations provide an important step in understanding the physical interactions between microbubbles and microvessels.
|
|
 |
|
Mechanisms for microvascular damage induced by ultrasound-activated microbubbles Chen, H., A. Brayman, A. Evan, and T. Matula,"Mechanisms for microvascular damage induced by ultrasound-activated microbubbles," Proceedings, International Society for Therapeutic Ultrasound Symposium 1481, New York, New York, U.S.A., 11-13 April, 41-46, doi:http://dx.doi.org/10.1063/1.4757308 (AIP, 2011). |
|
More Info
| |
|
11 Apr 2011 
|
|
 |
|
|
To provide insight into the mechanisms of microvascular damage induced by ultrasound-activated microbubbles, experimental studies were performed to correlate microvascular damage to the dynamics of bubble-vessel interactions. High-speed photomicrography was used to record single microbubbles interacting with microvessels in ex vivo tissue, under the exposure of short ultrasound pulses with a center frequency of 1 MHz and peak negative pressures (PNP) ranging from 0.8%u20134 MPa. Vascular damage associated with observed bubble-vessel interactions was either indicated directly by microbubble extravasation or examined by transmission electron microscopy (TEM) analyses. As observed previously, the high-speed images revealed that ultrasound-activated microbubbles could cause distention and invagination of adjacent vessel walls, and could form liquid jets in microvessels. Vessel distention, invagination, and liquid jets were associated with the damage of microvessels whose diameters were smaller than those of maximally expanded microbubbles. However, vessel invagination appeared to be the dominant mechanism for the damage of relative large microvessels.
|
|
 |
|
Blood vessel deformations on microsecond time scales by ultrasonic cavitation Chen, H., W. Kreider, A.A. Brayman, M.R. Bailey, and T.J. Matula, "Blood vessel deformations on microsecond time scales by ultrasonic cavitation," Phys. Rev. Lett., 106, 034301, doi:10.1103/PhysRevLett.106.034301, 2011. |
|
More Info
| |
|
18 Jan 2011 
|
|
 |
|
|
Transient interactions among ultrasound, microbubbles, and microvessels were studied using high-speed photomicrography. We observed liquid jets, vessel distention (motion outward against the surrounding tissue), and vessel invagination (motion inward toward the lumen). Contrary to current paradigms, liquid jets were directed away from the nearest vessel wall and invagination exceeded distention. These observations provide insight into the mechanics of bubble-vessel interactions, which appear to depend qualitatively upon the mechanical properties of biological tissues.
|
|
 |
|
Vascular damage by ultrasound-activated microbubble induced vessel invagination Chen, H., A. Brayman, A. Evan, and T. Matula, "Vascular damage by ultrasound-activated microbubble induced vessel invagination," Proceedings, IEEE International Ultrasonics Symposium, San Diego, USA, 11-14 October, 678-681, doi:10.1109/ULTSYM.2010.5935994 (IEEE, 2010). |
|
More Info
| |
|
11 Oct 2010 
|
|
 |
|
|
Vascular bioeffects produced by ultrasound contrast agent microbubbles are primarily manifested as damage to microvessels. The objective of this work is to directly observe the transient dynamics of bubble-vessel interactions and correlate the observed interactions with associated vascular damage. Microbubbles were perfused into microvessels in ex vivo rat mesenteries and then excited by a single 2 us long ultrasound pulse at 1 MHz. Meanwhile, 14 high-speed photomicrographic images were acquired using 50 ns shutter speeds. The targeted region was then examined by histology and transmission electron microscopy (TEM). Image registration was used to identify the specific vessels that the corresponding high-speed images were captured. The recorded high-speed images revealed that bubble-vessel interactions caused vessel wall distention (motion outward against the surrounding tissue) and invagination (motion inward toward the lumen). Invagination exceeding distention was observed in 60 out of 70 cases. Significant vessel invagination was correlated with vascular damage that was characterized by a separation of the endothelium from the surrounding tissue as revealed by both the histology and TEM analyses. The separation of the endothelium from the surrounding tissue is consistent with damage caused by tensile stresses at the vessel walls that lead to vessel invagination. This suggests that invagination may be an important mechanism by which microbubbles cause vascular damage.
|
|
 |
|
The peculiar interactions of microbubbles and microvessels Chen, H., A. Brayman, and T. Matula, "The peculiar interactions of microbubbles and microvessels," Proceedings, 20th International Congress on Acoustics, Sydney, Australia, 23-27 August, 1-5, (ICA, 2010). |
|
More Info
| |
|
23 Aug 2010 
|
|
 |
|
|
The application of microbubbles for both diagnostic macro- and molecular imaging and therapeudic ultrasound requires an understanding of the coupled interactions involving microbubble dynamics with the surrounding compliant microvessel. In this study, ultra-high speed microphotography was used to directly observe transient behaviors of microbubbles in microvessels of ex vivo rat mesenteries. Definity® microbubbles were perfused through the vasculature, and then excited by a 2-µs long ultrasound pulse with a center frequency of 1 MHz with peak negative pressures between 0.8-7.2 MPa. These amplitudes span the diagnostic to therapeutic pressure levels. During insonation, ultrahigh speed images were captured with 50-ns exposure time and 150-ns or 300-ns interframe time. The recorded images show a wonderful assortment of microbubble dynamics, including oscillation, translation, jetting, coalescence and fragmentation. These microbubble behaviors were coupled with the dynamic responses of the vessel wall, which showed distention, invagination, and even rupture.
|
|
 |
|
Blood vessel rupture by cavitation Chen, H., A.A. Brayman, M.R. Bailey, and T.J. Matula, "Blood vessel rupture by cavitation," Urol. Res., 38, 321-326, doi:10.1007/s00240-010-0302-5, 2010. |
|
More Info
| |
|
2 Aug 2010 
|
|
 |
|
|
Cavitation is thought to be one mechanism for vessel rupture during shock wave lithotripsy treatment. However, just how cavitation induces vessel rupture remains unknown. In this work, a high-speed photomicrography system was set up to directly observe the dynamics of bubbles inside blood vessels in ex vivo rat mesenteries. Vascular rupture correlating to observed bubble dynamics were examined by imaging bubble extravasation and dye leakage. The high-speed images show that bubble expansion can cause vessel distention, and bubble collapse can lead to vessel invagination. Liquid jets were also observed to form. Our results suggest that all three mechanisms, vessel distention, invagination and liquid jets, can contribute to vessel rupture.
|
|
 |
|
Observations of bubble-vessel interaction in ultrasound fields Chen, H., J. Kucewicz, W. Kreider, A. Brayman, M. Bailey, and T. Matula, "Observations of bubble-vessel interaction in ultrasound fields," Proceedings, IEEE International Ultrasonics Symposium, Rome, Italy, 20-23 September, 23-26, doi:10.1109/ULTSYM.2009.5441512 (IEEE, 2009). |
|
More Info
| |
|
20 Sep 2009 
|
|
 |
|
|
Interactions between bubbles and nearby boundaries have been studied for some time; however, the direct interactions between bubbles and tissue boundaries, especially blood vessel walls, have not been studied to a large extent. In this work highspeed microscopy was used to study the dynamical interaction between microbubbles and microvessels of ex vivo rat mesentery subjected to a single pulse of ultrasound. Ultrasound contrast agent microbubbles were injected into the blood vessels of rat mesentery subsequent to having the blood flushed out. India ink was used to increase the contrast between microvessels and surrounding tissues. Tissue samples were aligned at the focus of both an ultrasound transducer with a center frequency of 1 MHz and an inverted microscope coupled to a high speed camera. Fourteen high-speed microphotographic images were acquired for each experiment using 50 ns shutter speeds. Observations of the coupled dynamics between bubbles and vessels ranging from 10 micrometer to 100 micrometer diameters under the exposure of ultrasound of peak negative pressure within the range of 1 MPa to 7.8 MPa suggest that the vessel wall dilates during bubble expansion, and invaginates during bubble contraction. A significant finding is that the ratio of invagination to distension is usually >1 and large circumferential strains can be imposed on the vessel wall during vessel invagination. In addition, the surrounding tissue response was also quantified. Based on these studies, we hypothesize that vessel invagination is the dominant mechanism for the initial induction of vascular damage via cavitation.
|
|
 |
|
Microbubble dynamics in microvessels: Observations of microvessel dilation, invagination and rupture Chen, H., A. Brayman, and T. Matula, "Microbubble dynamics in microvessels: Observations of microvessel dilation, invagination and rupture," IEEE International Ultrasonics Symposium, Beijing, China, 2-5 November, 1163-1166, doi:10.1109/ULTSYM.2008.0280 (IEEE, 2008). |
|
More Info
| |
|
2 Nov 2008 
|
|
 |
|
|
Understanding the interaction of acoustically activated microbubbles with small blood vessels is important for designing better imaging schemes, and for targeting and drug delivery applications. To understand the fundamental mechanisms of this interaction, high-speed microscopy was used to observe microbubble dynamics in microvessels of ex vivo rat mesenteries exposed to a single pulse of ultrasound with a center frequency of 1 MHz and peak negative pressure (PNP) of 1.2 MPa or 11 MPa. It was found that microbubble oscillation caused adjacent microvessel dilation, invagination and even rupture on a microsecond time scale. In small microvessels, microbubble contacted with the vessel wall during expansion under both low and high pressure levels, and microvessel dilation generated by microbubble expansion was larger than invagination induced by bubble collapse. Specifically, under 11 MPa PNP insonation, a small microvessel (17 mum) dilated to 2.7times, and then invaginated to 0.4times of its original diameter, followed by extravasation of re-expanding daughter microbubbles indicating that the microvessel had been ruptured. For large microvessels, microbubbles did not contact with the vessel wall during expansion, and generated much less dilation than invagination at both pressure levels. In one case, a large microbubble caused the wall of a 100 mum microvessel to form a jet-like structure during invagination.
|
|
|
|
|
© 2011–2013 University of Washington
|
|